Mixing effects of Cr2O3–PrBaMn2O5 for increased redox cycling properties of Fe powder for a solid-oxide Fe–air rechargeable battery
Abstract
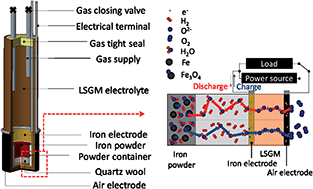
Large capacity rechargeable batteries are now strongly required for generating electric power from renewable energy, like solar cells or wind power generators. For this purpose, solid oxide Fe–air rechargeable batteries have been studied. In this study, for increasing the stability of the redox cycling properties of Fe powder at 623 K, mixing effects of Cr2O3 and PrBaMn2O5 catalysts were investigated and it was found that Fe powder mixed with 3 wt% Cr2O3–3 wt% PrBaMn2O5 showed excellent stability against redox cycling at 623 K. When Fe powder mixed with Cr2O3–PrBaMn2O5 was used, stable charge and discharge capacity could be achieved over 50 cycles by using the cell consisting of Ni–Fe/La0.9Sr0.1Ga0.8Mg0.2O3/Ba0.6La0.4CoO3. The discharge capacity of the cell achieved was larger than 770 mA h gFe−1 at 623 K. Increased capacity and cycle performance could be assigned to the deep redox degree of Fe powder.

 Please wait while we load your content...
Please wait while we load your content...