Rh-catalyzed aerobic oxidative cyclization of anilines, alkynes, and CO†
Abstract
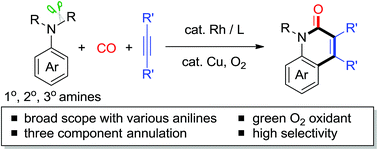
Transition-metal-catalyzed oxidative C–H cyclization of anilines has been an attractive and powerful strategy for the efficient construction of N-heterocycles. However, primary and tertiary anilines are rarely employed in this strategy due to the relative instability with strong oxidants or the presence of three C–N bonds. We describe here a novel Rh-catalyzed C–H cyclization of a wide range of anilines with alkynes and CO, using an aerobic oxidative protocol. Particularly, the simple primary anilines and readily prepared tertiary anilines could be easily converted to quinolin-2(1H)-ones, which are high value-added, biologically significant N-heterocycles, via C–N bond cleavage.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...