Porphyrinoid rotaxanes: building a mechanical picket fence†
Abstract
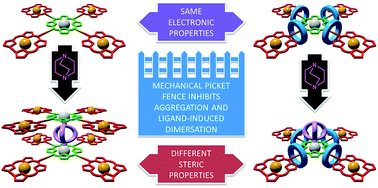
Building on recent progress in the synthesis of functional porphyrins for a range of applications using the Cu-mediated azide–alkyne cycloaddition (CuAAC) reaction, we describe the active template CuAAC synthesis of interlocked triazole functionalised porphyrinoids in excellent yield. By synthesising interlocked analogues of previously studied porphyrin–corrole conjugates, we demonstrate that this approach gives access to rotaxanes in which the detailed electronic properties of the axle component are unchanged but whose steric properties are transformed by the mechanical “picket fence” provided by the threaded rings. Our results suggest that interlocked functionalised porphyrins, readily available using the AT-CuAAC approach, are sterically hindered scaffolds for the development of new catalysts and materials.
- This article is part of the themed collection: International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS


 Please wait while we load your content...
Please wait while we load your content...