Photoredox ketone catalysis for the direct C–H imidation and acyloxylation of arenes†
Abstract
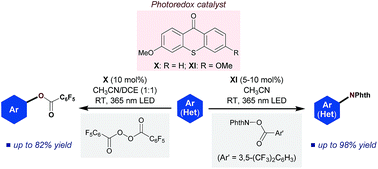
The photoexcited aryl ketone-catalyzed C–H imidation of arenes and heteroarenes is reported. Using 3,6-dimethoxy-9H-thioxanthen-9-one as a catalyst in combination with a bench-stable imidating reagent, C–N bond formation proceeds with high efficiency and a broad substrate scope. A key part of this method is that the thioxanthone catalyst acts as an excited-state reductant, thus establishing an oxidative quenching cycle for radical aromatic substitution. The synthetic potential of this photoexcited ketone catalysis is further demonstrated by application to the direct C–H acyloxylation of arenes.


 Please wait while we load your content...
Please wait while we load your content...