Supramolecular anion recognition in water: synthesis of hydrogen-bonded supramolecular frameworks†
Abstract
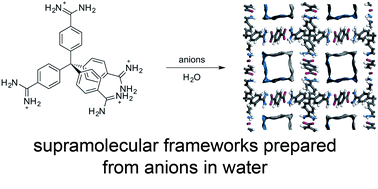
The interaction of tetratopic amidinium-containing receptors with terephthalate anions leads to porous framework materials assembled through charge-assisted hydrogen bonds. The frameworks form in good yield within minutes in water at room temperature, but no framework material is obtained if other anions (Cl−, Br−, NO3−, SO42− or isophthalate2−) are used in place of terephthalate. Two forms of the framework can be prepared: one with a connected pore network, and a more dense phase with discrete voids. We demonstrate that these are the kinetic and thermodynamic products, respectively. Either framework can be prepared independently and can be converted to the other form in response to stimuli. Furthermore, the frameworks can be controllably disassembled and reassembled in response to acid/base triggers suggesting that this new class of materials may have applications in the selective encapsulation and release of guests.
- This article is part of the themed collection: RACI100: Celebrating Australian Chemistry


 Please wait while we load your content...
Please wait while we load your content...