Copper(i)-catalyzed sulfonylative Suzuki–Miyaura cross-coupling†
Abstract
Using a simple copper(I) catalyst has allowed a high yielding sulfonylative-Suzuki–Miyaura cross-coupling reaction to be developed. The process provides a single step route to diaryl sulfones from the direct combination of aryl boronic acids, sulfur dioxide and aryl iodides, and represents the first sulfonylative variant of a classic cross-coupling reaction. Sulfur dioxide is delivered from the surrogate reagent, DABSO. Variation of the reaction conditions allowed interruption of the sulfonylative-Suzuki coupling, resulting in the formation of a presumed Cu–sulfinate intermediate. These sulfinates could be trapped as their sodium salts and treated with electrophiles to allow access to arylalkyl sulfones, β-hydroxyl sulfones, sulfonamides and sulfonyl fluorides.
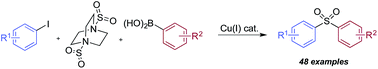
- This article is part of the themed collection: Most downloaded articles of 2017: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...