Triflimide-catalyzed allylsilane annulations of benzylic alcohols for the divergent synthesis of indanes and tetralins†
Abstract
The development of a triflimide-catalyzed annulation of benzylic alcohols with allylsilanes for the synthesis of indane or tetralin structures is reported. In this fragment coupling reaction, complexity is built rapidly from readily available starting materials to yield diverse sets of products with up to three contiguous stereocenters. Indanes or tetralins can be generated from common precursors depending on the structure of the allylsilane reagent used. The concise synthesis of several lignan natural products highlights the utility of this newly devised methodology.
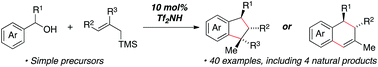


 Please wait while we load your content...
Please wait while we load your content...