Reagent-controlled enantioselectivity switch for the asymmetric fluorination of β-ketocarbonyls by chiral primary amine catalysis†
Abstract
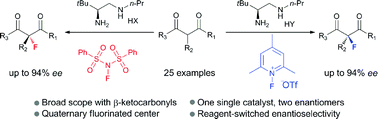
A reagent-controlled enantioselectivity switch was uncovered in the asymmetric α-fluorination of β-ketocarbonyls by a chiral primary amine catalyst. By a simple swap of fluorination reagents, both enantiomers of the quaternary fluorination adducts could be obtained with good yields and high enantioselectivity. Mechanistic studies disclosed dual H-bonding and electrostatic stereocontrolling modes for the catalysis.

 Please wait while we load your content...
Please wait while we load your content...