Light-induced decarboxylation in a photo-responsive iron-containing complex based on polyoxometalate and oxalato ligands†
Abstract
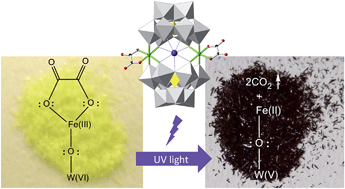
A new photoresponsive molecular polyanion in which two Fe(III) ions are simultaneously coordinated by two [A-α-PW9O34]9− polyoxometalate units and two oxalato ligands has been obtained. When irradiated with UV light its potassium salt, 1, exhibits a remarkable photocoloration effect, attributable to the partial reduction of the POM units to give rise to a mixed-valence species. The photoinduced process is intramolecular and involves electron transfer from the oxalato ligands, which partially decompose releasing CO2, towards the Fe(III) and the POM. This mechanism has been confirmed by DRS, IR, XPS and Mössbauer spectroscopy, magnetism and elemental analysis. An analogous derivative of 1 containing malonato ligands does not exhibit such photoactive behaviour, which is evidence that the oxalate ligand is essential for the photoactivity of 1. To our knowledge, 1 represents the first POM-based compound in which the photocoloration effect does not require the presence of intermolecular short interactions.


 Please wait while we load your content...
Please wait while we load your content...