Precision polymers containing main-chain-amino acids: ADMET polymerization and crystallization†
Abstract
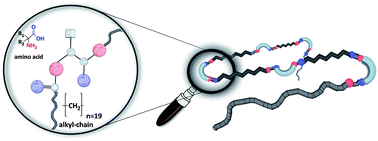
The synthesis of new poly(alkyl)-precision oligomers and polymers displaying different types of amino acids (chiral/achiral, polar/non-polar) placed at every 19th carbon atom are presented. Placing ω-alkenyl moieties onto appropriately N- and C-terminus functionalized amino acids successfully enabled ADMET-polymerization of these monomers. Grubbs 1st catalyst under melt-polycondensation-conditions was found to be the most effective with respect to the obtained molecular weights of the polymers, the isomerization of the olefins and their final yield, yielding molecular weights up to ∼22 kDa. The obtained polymers and the subsequent hydrogenation of the double bonds with p-toluenesulfonhydrazide (TsNHNH2) was proven by NMR, GPC, MALDI-ToF-MS and IR measurements. Investigation of the thermal behavior of the monomers and polymers via DSC measurements displays amorphous structures for monomers and polymers with unpolar amino acid side chains, whereas for polymers bearing the polar glutamic- and aspartic acid moieties crystalline morphologies are observed. An ordered lamellar crystal phase is observed where the amino acid branches are either incorporated or excluded from the unit cell, as proven by WAXS data.


 Please wait while we load your content...
Please wait while we load your content...