Energy level alignment at semiconductor–water interfaces from atomistic and continuum solvation models†
Abstract
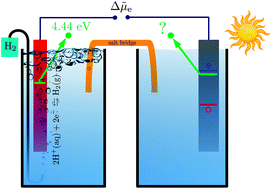
Accurate and efficient methods for predicting the alignment between a semiconductor's electronic energy levels and electrochemical redox potentials are needed to facilitate the computational discovery of photoelectrode materials. In this paper, we present an approach that combines many-body perturbation theory within the GW method with continuum solvation models. Specifically, quasiparticle levels of the bulk photoelectrode are referenced to the outer electric potential of the electrolyte by calculating the change in electric potential across the photoelectrode–electrolyte and the electrolyte–vacuum interfaces using continuum solvation models. We use this method to compute absolute energy levels for the prototypical rutile (TiO2) photoelectrode in contact with an aqueous electrolyte and find good agreement with predictions from atomistic simulations based on molecular dynamics. Our analysis reveals qualitative and quantitative differences of the description of the interfacial charge density in atomistic and continuum solvation models and highlights the need for a consistent treatment of electrode–electrolyte and electrolyte–vacuum interfaces for the determination of accurate absolute energy levels.


 Please wait while we load your content...
Please wait while we load your content...