Facile synthesis of novel hybrid POSS biomolecules via “Click” reactions†
Abstract
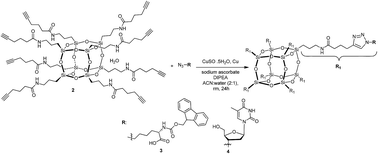
A novel alkyne-terminated cubic-octameric POSS was synthesised in high yield (82–90%). The X-ray crystal structure revealed intra- and intermolecular hydrogen bonding between the amide groups of the arms. Hybrid biomaterials were synthesised in nearly quantitative yields via a click reaction with (i) azido-N-Fmoc-norleucine and (ii) 3′-azido-3′-deoxythymidine.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC


 Please wait while we load your content...
Please wait while we load your content...