Bifunctional electro-catalytic performances of CoWO4 nanocubes for water redox reactions (OER/ORR)
Abstract
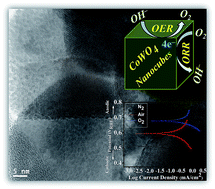
In this paper, we report the synthesis of cube shaped nanoparticles of CoWO4 (∼30 nm) by molten salts and their bifunctional electro-catalytic activities in water redox reactions for oxygen evolution and oxygen reduction reactions (OER and ORR). Bifunctional performances of CoWO4 nano-cubes are explored for water electrolysis in an alkaline medium (1.0 M KOH) vs. reversible hydrogen electrode (RHE) under various atmospheres (N2, air and O2). Low overpotential (η10 = 0.45 V) of CoWO4 nano-cubes is accomplished at the current density of 10 mA cm−2. Tafel polarization curves (potential vs. log current density) reveal relatively lower slope values for OER (∼82 mV dec−1) and ORR (∼68 mV dec−1) compared to previous reports. Stability test of electrode materials has been performed using chrono-amperometry (CA) at fixed potential for 500 seconds. Kinetics and mobility of electrons have also been studied during the water redox reactions. Stable nature and enhanced bifunctional electro-catalytic performances of earth abundant CoWO4 electrodes could be used as the replacement of expensive electroactive noble electrode materials (Pt, Ir, Ru etc.) for water electrolysis (OER and ORR) in near future.


 Please wait while we load your content...
Please wait while we load your content...