Binder free 2D aligned efficient MnO2 micro flowers as stable electrodes for symmetric supercapacitor applications†
Abstract
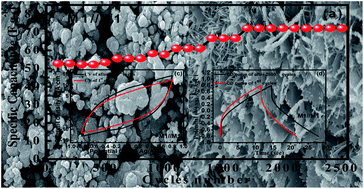
Herein, δ-MnO2 micro-flower thin films are grown directly onto a stainless steel mesh via a simple rotational chemical bath deposition technique. Moreover, the influence of the concentration of precursor ratio of MnSO4 : KMnO4 is investigated and the obtained samples are designated as M1 (KMnO4 : MnSO4 = 3 : 1), M2 (KMnO4 : MnSO4 = 3 : 2) and M3 (KMnO4 : MnSO4 = 3 : 3). The concentration of MnSO4 as a starting material has a significant influence on the reaction kinetics, which subsequently alters the morphology and also the electrochemical performance. Among these three electrodes, the M1 electrode exhibits a high specific capacitance of 376 F g−1 at a current density of 5 mA cm−2 and a high specific energy of 52 W h kg−1, which is higher than M2 (specific capacitance 312 F g−1 and specific energy 43 W h kg−1) and M3 (specific capacitance 283 F g−1 and specific energy 39 W h kg−1) electrodes. Due to the interesting performance of the M1 based electrode, the symmetric device is fabricated using two electrodes M1 (3 : 1) and represented as SSM/M1//M1/SSM. The device provides a maximum specific capacitance of 87 F g−1 and specific energy density of 32 W h kg−1 at a current density of 5 mA cm−2. In addition, the symmetric device of the M1 electrode also exhibits good cycle stability showing 138% capacitance retention up to 2500 cycles. The enhanced electrochemical performance could be attributed to the direct growth of micro-flowers of MnO2 on a stainless steel mesh, which provides more pathways for easy diffusion of electrolyte ions into the electrode. This study provides new insight and pathways for the development of low-cost and high-performance energy storage devices.


 Please wait while we load your content...
Please wait while we load your content...