Ferroelectric polarization of hydroxyapatite from density functional theory
Abstract
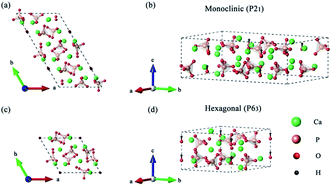
The theoretical ferroelectric polarization of the low-temperature (monoclinic, P21) phase and the high-temperature (hexagonal, P63) phase of hydroxyapatite Ca10(PO4)6(OH)2 is calculated based on the density functional theory (DFT). In the monoclinic structure, the value of ferroelectric polarization is found to be 9.87 μC cm−2 along the [001] direction. In the hexagonal structure, the ferroelectric polarization is 7.05 μC cm−2 along the [001] direction. The main contribution to the electric polarization comes from ordered hydroxyl OH− anions for both phases, although the inorganic Ca5(PO4)3 apatite framework also gives a non-negligible contribution. A detailed analysis of ferroelectric polarization and structural change of the hydroxyapatite is presented for a better understanding of this important biomaterial.


 Please wait while we load your content...
Please wait while we load your content...