Dually sensitive dextran-based micelles for methotrexate delivery†
Abstract
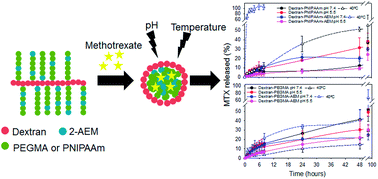
Temperature-sensitive polymeric micelles were prepared from dextran grafted with poly(N-isopropylacrylamide) (PNIPAAm) or polyethylene glycol methyl ether (PEGMA) via controlled radical polymerization and evaluated as delivery systems of the anticancer drug methotrexate (MTX). Polymer-grafting was carried out after introduction of initiating groups onto the polysaccharide backbone, without the need for protection of hydroxyl groups and avoiding the use of toxic solvents. Temperature-responsive dextran-based copolymers were designed to exhibit self-aggregation behaviour, affinity for MTX and high cellular internalization. In addition, some grafted polymers incorporated 2-aminoethyl methacrylate to reinforce MTX encapsulation in the micelles by means of ionic interactions. Dextran-based micelles were cytocompatible and had an appropriate size to be used as drug carriers. MTX release was dependent on the pH and temperature. The combination of poly(2-aminoethylmethacrylate) and PNIPAAm with the dextran backbone permitted the complete release of MTX at normal physiological temperature. Co-polymer micelles were highly internalized by tumour cells (CHO-K1) and, when loaded with MTX, led to enhanced cytotoxicity compared to the free drug.


 Please wait while we load your content...
Please wait while we load your content...