Ir-phosphate cocatalyst for photoelectrochemical water oxidation using α-Fe2O3†
Abstract
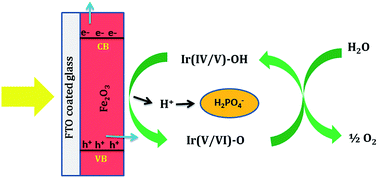
α-Fe2O3 is an ideal photoanode for solar water oxidation owing to its visible light absorbing capability, suitable valence band position, low cost, high abundance, non-toxicity and eco-friendliness. However, the reported efficiencies are very low due to the poor kinetics of water oxidation by photogenerated holes on α-Fe2O3. In the present study, an α-Fe2O3 electrode is obtained by heating a film of Fe which is prepared by the electrochemical reduction of Fe2+ ions. Film thickness and calcination temperature are carefully optimized to get a maximum photoresponse in neutral phosphate solution. In order to improve the water oxidation kinetics and reduce the charge carrier recombination, an iridium-phosphate (Ir-Pi) catalyst is electrodeposited on the surface of α-Fe2O3. Ir-Pi is found to reduce the OER onset potential by 350 mV, and enhances the photocurrent density by 3 times at 1.23 V vs. RHE.


 Please wait while we load your content...
Please wait while we load your content...