Unusual H/D isotope effect in isomerization and keto–enol tautomerism reactions of pyruvic acid: nuclear quantum effect restricts some rotational isomerization reactions†
Abstract
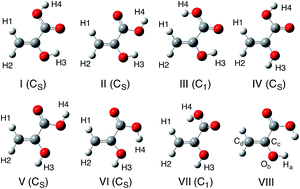
Isomerization and keto–enol tautomerism reactions of the pyruvic acid molecule have been investigated using the multicomponent B3LYP (MC_B3LYP) methods, which can take account of the nuclear quantum effect (NQE) of a light nucleus, such as a proton and a deuteron. While the conventional harmonic zero point vibrational energy (ZPVE) correction makes the activation energies of all the reactions in this system lower, a contrasting behavior is found in our MC_B3LYP results for several rotational reactions. In such cases, the H/D isotope effect on the activation energy is also completely opposite between harmonic ZPVE-corrected B3LYP and MC_B3LYP calculations. In our MC_B3LYP calculation, the activation energies of several C–C or O–H rotational reactions of H species are slightly higher than those of D species, since the NQE of a hydrogen-bonded proton strengthens the hydrogen-bonded interaction more than that of a deuteron, and, thus, the rotational motion of H species is restricted. Such an “unusual” H/D isotope effect on the activation energies can be observed only in the MC_B3LYP results. Our MC_B3LYP calculations clearly demonstrate that direct inclusion of NQE is indispensable to analyze H/D isotope effects on activation energies of not only hydrogen transfer reactions but also C–C and O–H rotational reactions in the isomerization and keto–enol tautomerism of pyruvic acid molecule.


 Please wait while we load your content...
Please wait while we load your content...