Reaction of the hydrogen atom with nitrous oxide in aqueous solution – pulse radiolysis and theoretical study†
Abstract
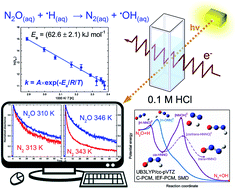
UB3LYP/cc-pVTZ computations using C-PCM, IEF-PCM, and SMD water-solvent models have been performed for the reaction of the H˙ atom with nitrous oxide (N2O) producing N2 and ˙OH in aqueous solution. The H˙ atom attacks the oxygen atom in the N2O molecule resulting in the formation of the [H–ONN]‡ transition state and its decomposition into ˙OH and N2. This direct path requires 54.2 kJ mol−1 (PCM) or 54.6 kJ mol−1 (SMD) compared to 53.0 kJ mol−1 in a vacuum. The H˙ atom addition to the nitrogen end leads to the [H–NNO]‡ transition state decaying to a cis-HNNO intermediate that after transformation to [NNOH]‡ finally produces ˙OH and N2. The total energy expense associated with the indirect mechanism, 67.6 kJ mol−1 (PCM) or 65.5 kJ mol−1 (SMD), is slightly smaller compared to 67.7 kJ mol−1 computed for the reaction in vacuum. The temperature dependence of the reaction rate constant obtained based on the pulse radiolysis measurements in N2O-saturated 0.1 M HCl solution over the temperature range of 296–346 K shows the activation energy (62.6 ± 2.1) or (59.9 ± 2.1) kJ mol−1 depending on a form of the pre-exponential factor in the Arrhenius equation, A or A′ × T, respectively. The activation energy, almost three times higher than observed in gases at temperatures below 500 K, indicates predominance of the direct reaction path via [H–ONN]‡. The indirect mechanism may also contribute, but in contrast to the gas phase reaction neither tunnelling from [H–NNO]‡ to [NNOH]‡ nor collisional stabilization of [H–NNO]‡ occurs in solution.


 Please wait while we load your content...
Please wait while we load your content...