Oxidation of diazenyl-protected N-heterocycles – a new entry to functionalized lactams†
Abstract
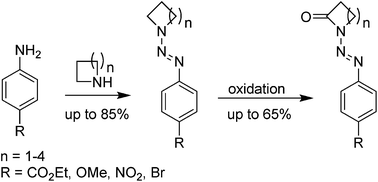
Functionalized lactams are an important class of heterocycles since they are useful intermediates in organic synthesis and show biological activity in diverse therapeutic applications. In the herein presented study, a strategy for the synthesis of N-aryldiazenyllactams, offering the direct access to protected lactam derivatives is described. After the formation of triazenes from diazonium salts and commercially available N-heterocycles, oxidation (directed CH activation) with periodate under ruthenium catalysis furnished N-diazenyllactams in one step. To demonstrate the suitability of the resulting lactams for further functionalizations, the alkylation of a N-diazenyllactam is presented for one example.


 Please wait while we load your content...
Please wait while we load your content...