Solvent-free Knoevenagel reaction catalysed by reusable pyrrolidinium base protic ionic liquids (PyrrILs): synthesis of long-chain alkylidenes†
Abstract
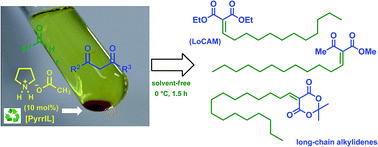
In this work, an efficient and reusable pyrrolidinium ionic liquid (PyrrIL) catalysis system was developed and used in a Knoevenagel condensation reaction of long-chain aldehydes with several 1,3-dicarbonyl compounds. The Knoevenagel condensation promoted by the PyrrILs proceeded smoothly and cleanly in solvent-free conditions, yielding good quantities of the condensation products, long-chain alkylidenes. Moreover, this catalysis system was recyclable at least four times, and no significant loss of activity was observed. This protocol has notable advantages, such as ease of workup and convenient reuse of the ionic liquid, which could help reduce disposal costs and contribute to the development of new catalysts in chemical processes.


 Please wait while we load your content...
Please wait while we load your content...