Superlubricity of hydrogenated carbon films in a nitrogen gas environment: adsorption and electronic interactions at the sliding interface
Abstract
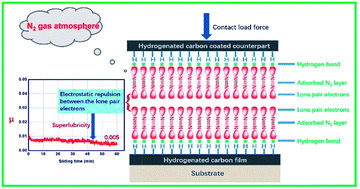
The tribological behavior of hydrogenated amorphous carbon film (a-C:H) is known to be very sensitive to the external environment. In an N2 environment, a-C:H can maintain long-term superlubricity. Currently, no convincing explanation is available for the mechanism through which N2 significant decreases the friction coefficients. In this work, we explain the tribological behavior of a-C:H films by accounting for the internal electronic structure of ambient gas molecules, their atomic electronegativity, the surface bonding state of a-C:H, and the synergistic effects of these factors. Here, we proposed a mechanism based on the unique electronic structure of N2 molecules. N2 has two lone electron pairs, and its lone-pair electrons interact with surface C–H bonds of a-C:H, forming –C–H⋯:N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: interactions that are similar to the hydrogen bonds in water. This causes N2 to be adsorbed on the film's surface. As two surfaces approach each other, the other lone-pair electrons in N2 become close, generating electrostatic repulsion and resulting in super-low friction. Based on the above analysis, we calculated the friction forces of a self-mated a-C:H film in different environments (vacuum, N2, O2) using density functional theory. These theoretical calculations matched experimental results, indicating that the proposed approach is reasonable.
N: interactions that are similar to the hydrogen bonds in water. This causes N2 to be adsorbed on the film's surface. As two surfaces approach each other, the other lone-pair electrons in N2 become close, generating electrostatic repulsion and resulting in super-low friction. Based on the above analysis, we calculated the friction forces of a self-mated a-C:H film in different environments (vacuum, N2, O2) using density functional theory. These theoretical calculations matched experimental results, indicating that the proposed approach is reasonable.

 Please wait while we load your content...
Please wait while we load your content...