Copper-assisted azide–alkyne cycloaddition chemistry as a tool for the production of emissive boron difluoride 3-cyanoformazanates†
Abstract
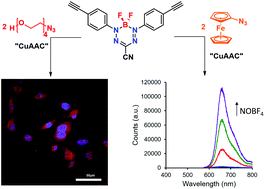
The synthesis and characterization of emissive boron difluoride (BF2) complexes of 3-cyanoformazanate ligands produced using copper-assisted azide–alkyne cycloaddition (CuAAC) chemistry is described. Detailed spectroscopic and electrochemical characterization of benzyl-functionalized complexes served as models and demonstrated that triazole formation at the N-aryl substituents of the formazanate ligand scaffold led to red-shifted absorption and emission maxima and more difficult electrochemical reduction compared to alkyne-substituted precursors. CuAAC chemistry was also used to append ferrocene and tetraethylene glycol substituents to the formazanate backbone. In the case of the ferrocene-substituted complexes, fluorescence was quenched and a reversible oxidation feature (in addition to the reduction features associated with formazanate complexes) was observed using cyclic voltammetry. Treatment with NOBF4 oxidized ferrocene to ferrocenium and resulted in the reestablishment of fluorescence. Tetraethylene glycol substitution produced the first water soluble BF2 formazanate, which was shown to distribute throughout the cytoplasm and nucleus of mouse fibroblast cells when studied as a fluorescence imaging agent.

 Please wait while we load your content...
Please wait while we load your content...