Trimerization of enones under air enabled by NHC/NaOtBu via a SET radical pathway†
Abstract
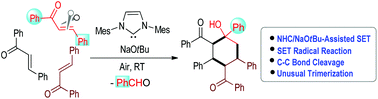
An unusual trimerization of enone via a formal [2 + 2 + 2] process is disclosed. The reaction is initiated by a radical process enabled by NaOtBu and N-heterocyclic carbene (NHC). Molecular oxygen (air) is involved in key steps of the radical intermediate formation and carbon–carbon bond cleavage in this trimerization reaction to form highly substituted cyclohexane derivatives. In previous studies, alkali metal tert-butoxides such as NaOtBu were mainly employed to generate radical intermediates from aryl halides. Here we provide a new avenue in using NaOtBu and combined NHC/NaOtBu to generate radical intermediates from enones for further reactions.

 Please wait while we load your content...
Please wait while we load your content...