Cyanopyridone flanked the tetraphenylethylene to generate an efficient, three-dimensional small molecule non-fullerene electron acceptor†
Abstract
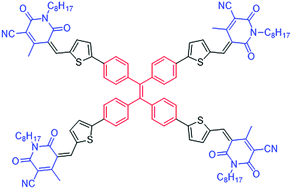
Herein we report the design, synthesis and characterization of a novel material, (5Z,5′Z,5′′Z,5′′′E)-5,5′,5′′,5′′′-(((ethene-1,1,2,2-tetrayltetrakis(benzene-4,1-diyl))tetrakis(thiophene-5,2-diyl))tetrakis(methanylylidene))tetrakis(4-methyl-1-octyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile) (TPE-CP4), which was generated using a combination of tetraphenylethylene and cyanopyridone functionalities. The tetraphenylethylene unit was terminally functionalized with cyanopyridone in order to generate a three-dimensional molecular architecture. The new material TPE-CP4 was produced via a Knoevenagel condensation reaction and was found to be highly soluble in a variety of common organic solvents, such as chloroform and o-dichlorobenzene. TPE-CP4 exhibited promising optoelectronic properties and energy levels complementing those of the conventional donor polymers poly(3-hexylthiophene) [P3HT] and Poly({4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl}{3-fluoro-2-[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl}) [PTB7]. Due to its optoelectronic properties, solubility and good film forming capability, TPE-CP4 was incorporated as an n-type semiconducting component along with the commercially available P3HT and PTB7 as the p-type semiconductors in BHJ photovoltaic devices. TPE-CP4 performed very well with both of the donor polymers and afforded 6.02% and 6.72% power conversion efficiencies with P3HT and PTB7, respectively. Not only is TPE-CP4 the first reported example combining tetraphenylethylene and cyanopyridone structural units, but the device performance indicates that it is an efficient non-fullerene acceptor.


 Please wait while we load your content...
Please wait while we load your content...