Gold and nickel alkyl substituted bis-thiophenedithiolene complexes: anionic and neutral forms†
Abstract
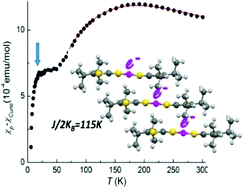
The Au and Ni monoanionic complexes of tert-butyl and diisopropyl substituted thiophenedithiolate ligands, [M(α-tb-tpdt)2] and [M(α-dp-tpdt)2], were synthesized and characterized namely by single crystal X-ray diffraction and magnetic susceptibility measurements. These complexes, prepared in a first step as monoanionic species, are easier to oxidize than the related non-substituted thiophenedithiolates and could be obtained also as stable neutral species. As expected, the peripheral alkyl groups in the ligands also confer a high solubility to the complexes in common organic solvents. The neutral gold complex [Au(α-tb-tpdt)2] presents a significant ligand asymmetry indicative of unpaired electron localization in one ligand at variance with [Au(α-dp-tpdt)2] that is within experimental uncertainty fully symmetric illustrating the role of intermolecular interactions in the stabilization of SOMO⋯SOMO interactions. While in [Au(α-tb-tpdt)2] a significant intermolecular interaction between paramagnetic molecules is possible leading to diamagnetic dimers of molecules, in [Au(α-dp-tpdt)2] the bulkier substituents prevent the intermolecular interactions, leading to a regular stacking of molecules in symmetrical configuration. The regular stacks of paramagnetic [Au(α-dp-tpdt)2] units behave, at high temperatures, as antiferromagnetic chains undergoing an AFM transition at ca. 25 K.


 Please wait while we load your content...
Please wait while we load your content...