Exploration of photophysics of 2,2′-pyridil at room temperature and 77 K: a combined spectroscopic and quantum chemical approach†
Abstract
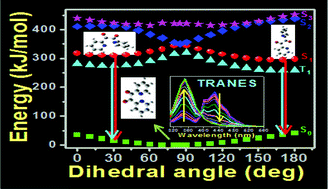
The photophysics of 2,2′-pyridil has been explored thoroughly using steady state and time resolved fluorometric techniques at room temperature (RT) in liquid media as well as in glassy matrices at cryogenic temperature (77 K). Ethanol and methylcyclohexane are exploited for this purpose, as polar and non-polar media respectively. Notwithstanding the observation of multiple emissions from the fluorophore, the experiments unequivocally rule out emission from excited singlet states other than the S1 state, consistent with Kasha's rule. Among 1,2-dicarbonyl molecular systems, this behavior resembles that of α-furil, while it contradicts that of benzil and α-naphthil which exhibit S2 emissions. The dual fluorescence and dual phosphorescence of the fluorophore are ascribed to the emissions originating from the two conformers, namely near-trans and relaxed skew. Coexistence of the two conformers is substantiated by time resolved area normalized emission spectroscopy (TRANES) at both RT and 77 K. The potential energy curves (PECs) simulated from calculations based on density functional theory and its time dependent extension provide adequate support to the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...