Universal access to megastigmanes through controlled cyclisation towards highly substituted cyclohexenes†
Abstract
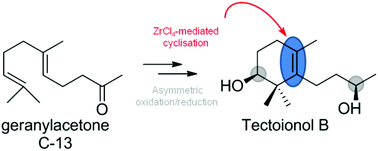
We report the selective formation of cyclohexenes with a tetrasubstituted double bond, the structural key element of megastigmanes. For this purpose the ZrCl4-mediated epoxide ring opening of epoxy-geranylacetone was employed. This approach provides a universal entry to the preparation of the members of the megastigmane family, which was exemplified in the asymmetric synthesis of tectoionol B.

 Please wait while we load your content...
Please wait while we load your content...