Enediyne-based protein capture agents: demonstration of an enediyne moiety acting as a photoaffinity label†
Abstract
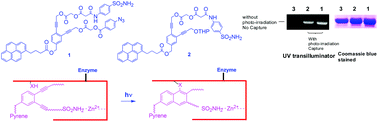
Two enediyne based protein-capture compounds 1 and 2 were synthesized. Both these molecules have an aryl sulfonamide for reversible binding with Human Carbonic Anhydrase II (HCA II) and a pyrene moiety for the visualization of a capture event. While compound 1 has an aryl azide as a photo cross-linking agent, compound 2 lacks the azide moiety. Capture experiments with HCA II however show that both 1 and 2 can photo cross-link with the protein as indicated in gel electrophoresis as well as MALDI analysis after tryptic digestion of HCA II. This observation demonstrates the ability of the enediyne moiety to act as a photo-affinity label possibly via the addition of nucleophilic amino acids to the partially zwitterionic singlet form of the diradical generated by photo Bergman cyclization.

 Please wait while we load your content...
Please wait while we load your content...