A nickel catalyzed acceptorless dehydrogenative approach to quinolines†
Abstract
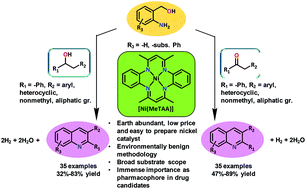
A general, efficient and environmentally benign, one-step synthesis of substituted quinoline derivatives was achieved by acceptorless dehydrogenative coupling of o-aminobenzylalcohols with ketones and secondary alcohols catalyzed by a cheap, earth abundant and easy to prepare nickel catalyst [Ni(MeTAA)], featuring a tetraaza macrocyclic ligand (tetramethyltetraaza[14]annulene (MeTAA)). A wide variety of substituted quinolines were synthesized in high yields starting from readily available o-aminobenzylalcohols and ketones or secondary alcohols. A few controlled reactions were carried out to establish the acceptorless dehydrogenative nature of the reactions.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...