Regioselective and enantiospecific synthesis of the HSP co-inducer arimoclomol from chiral glycidyl derivatives†
Abstract
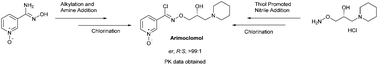
A new efficient chiral synthesis of enantiopure arimoclomol (2) is reported from (R)-(−)-glycidyl nosylate (11) with complete retention of chiral integrity. Off-target pharmacology of arimoclomol (2) was evaluated against a representative set of drug targets and showed modest binding to a few kinases. Pharmacokinetic data was generated in vivo in mouse and showed a low brain : plasma ratio. These studies will be helpful towards a better understanding of the PK-PD relationship of 2 in disease models.


 Please wait while we load your content...
Please wait while we load your content...