In silico analyses of essential interactions of iminosugars with the Hex A active site and evaluation of their pharmacological chaperone effects for Tay–Sachs disease
Abstract
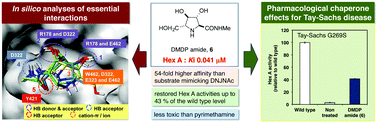
The affinity of a series of iminosugar-based inhibitors exhibiting various ring sizes toward Hex A and their essential interactions with the enzyme active site were investigated. All the Hex A-inhibiting iminosugars tested formed hydrogen bonds with Arg178, Asp322, Tyr421 and Glu462 and had the favorable cation–π interaction with Trp460. Among them, DMDP amide (6) proved to be the most potent competitive inhibitor with a Ki value of 0.041 μM. We analyzed the dynamic properties of both DMDP amide (6) and DNJNAc (1) in aqueous solution using molecular dynamics (MD) calculations; the distance of the interaction between Asp322 and 3-OH and Glu323 and 6-OH was important for stable interactions with Hex A, reducing fluctuations in the plasticity of the active site. DMDP amide (6) dose-dependently increased intracellular Hex A activity in the G269S mutant cells and restored Hex A activity up to approximately 43% of the wild type level; this effect clearly exceeded the border line treatment for Tay–Sachs disease, which is regarded as 10–15% of the wild type level. This is a significantly greater effect than that of pyrimethamine, which is currently in Phase 2 clinical trials. DMDP amide (6), therefore, represents a new promising pharmacological chaperone candidate for the treatment of Tay–Sachs disease.


 Please wait while we load your content...
Please wait while we load your content...