Selective lysine modification of native peptides via aza-Michael addition†
Abstract
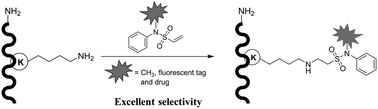
A series of vinylsulfonamides were synthesized and screened for site-selective modification of the ε-amino group of lysine-bearing free α-amine residues. N-Methyl-N-phenylethenesulfonamide has emerged as an applicable reagent and has been developed for efficient and highly selective modification of the lysine residue of native peptides in the presence of a free N-terminus via aza-Michael addition. We demonstrated that functional N-phenylvinylsulfonamide derivatives with a fluorescent moiety or drug could also be conjugated to the lysine residue of octreotide and insulin with high specificity, without modifying the N-terminus. Our method provides a promising strategy for site-selective lysine functionalization in native peptides with a free N-terminus.


 Please wait while we load your content...
Please wait while we load your content...