An expedient synthesis of flexible nucleosides via a regiocontrolled enzymatic glycosylation of functionalized imidazoles†
Abstract
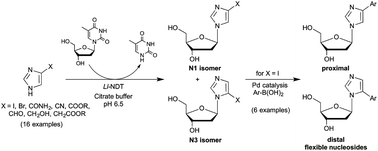
A versatile two-step synthesis of C4- and C5-arylated 2′-deoxyribosylimidazoles was elaborated using enzymatic N-transglycosylation followed by microwave-assisted Pd-catalysed arylation reactions. We report herein the reaction conditions that permit managing regioselectivity (N3 versus N1-isomers) in the enzymatic glycosylation of 4-iodoimidazole using the nucleoside N-deoxyribosyltransferase from L. leichmannii. Regiocontrolled glycosylation was also observed among several other imidazole derivatives studied, providing simple access to isomers not readily accessible by chemical routes. Finally, a series of flexible nucleosides was obtained in one step from 4- or 5-iodo-imidazole nucleosides by the Suzuki–Miyaura cross-coupling reaction with (hetero)aryl-boronic acids in aqueous media. Moreover, this chemoenzymatic approach is compatible with a one-pot two-step process affording a straightforward access to a broad array of potential anticancer and antiviral drugs as well as new DNA building blocks.


 Please wait while we load your content...
Please wait while we load your content...