A study of synthetic approaches to 2-acyl DHA lysophosphatidic acid†
Abstract
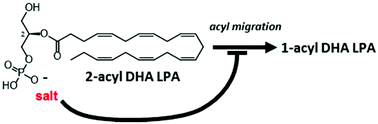
Lysophosphatidic acid (LPA) is a chemical mediator with a very simple glycerophospholipid structure. 1-Acyl LPA and 2-acyl LPA are biosynthesized in vivo. Unlike 1-acyl LPA, the biological function of 2-acyl LPA has been hardly elucidated and even organic synthesis of 2-acyl LPA had not been established. We suppressed acyl migration by formation of a salt with a phosphate group in order to synthesize 2-acyl LPA condensed with docosahexaenoic acid.


 Please wait while we load your content...
Please wait while we load your content...