Oxidative coupling of Michael acceptors with aryl nucleophiles produced through rhodium-catalyzed C–C bond activation†
Abstract
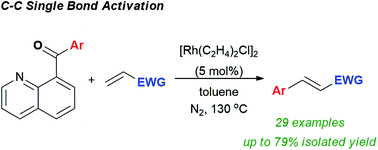
Utilizing rhodium catalysis, aryl nucleophiles generated via carbon–carbon single bond activation successfully undergo oxidative coupling with Michael acceptors. The reaction scope encompasses a broad range of nucleophiles generated from quinolinyl ketones as well as a series of electron deficient terminal alkenes, illustrating the broad potential of intersecting carbon–carbon bond activation with synthetically useful coupling methodologies. The demonstrated oxidative coupling produces a range of cinnamyl derivatives, several of which are challenging to prepare via conventional routes.


 Please wait while we load your content...
Please wait while we load your content...