A sialosyl sulfonate as a potent inhibitor of influenza virus replication†
Abstract
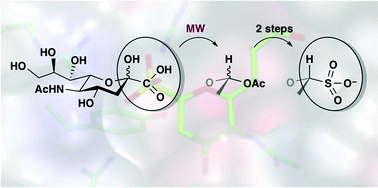
A new direction for influenza virus sialidase inhibitor development was identified using a sulfonate congener of 2-deoxy-2-β-H N-acetylneuraminic acid. Sialosyl sulfonates can be synthesised efficiently in four steps from N-acetylneuraminic acid via a microwave assisted decarboxylation. The presence of the sulfonate group significantly increases inhibition of influenza virus sialidase and viral infection when compared to the carboxylate congener, and also to the benchmark sialidase inhibitor 2,3-dehydro-2-deoxy-N-acetylneuraminic acid, Neu5Ac2en.


 Please wait while we load your content...
Please wait while we load your content...