Tandem SN2′ nucleophilic substitution/oxidative radical cyclization of aryl substituted allylic alcohols with 1,3-dicarbonyl compounds†
Abstract
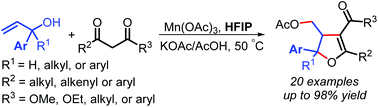
A novel and efficient tandem SN2′ nucleophilic substitution/oxidative radical cyclization reaction of aryl substituted allylic alcohols with 1,3-dicarbonyl compounds has been developed by using Mn(OAc)3 as an oxidant, which enables the expeditious synthesis of polysubstituted dihydrofuran (DHF) derivatives in moderate to high yields. The use of weakly acidic hexafluoroisopropanol (HFIP) as the solvent rather than AcOH has successfully improved the yields and expanded the substrate scope of this type of radical cyclization reactions. Mechanistic studies confirmed the cascade reaction process involving a final radical cyclization.


 Please wait while we load your content...
Please wait while we load your content...