Copper-catalysed enantioselective Michael addition of malonic esters to β-trifluoromethyl-α,β-unsaturated imines†
Abstract
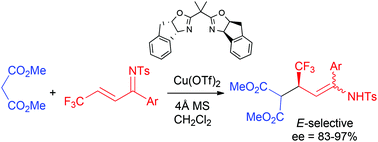
Copper triflate-BOX complexes catalyse the enantioselective conjugate addition of methyl malonate to β-trifluoromethyl-α,β-unsaturated imines to give the corresponding enamines bearing a trifluoromethylated stereogenic centre with good yields, and diastereo- and enantioselectivities. The usefulness of the method has been shown with the synthesis of optically active β-trifluoromethyl δ-amino esters and optically active trifluoromethyl piperidones.


 Please wait while we load your content...
Please wait while we load your content...