Protecting group free synthesis of glycosyl thiols from reducing sugars in water; application to the production of N-glycan glycoconjugates†
Abstract
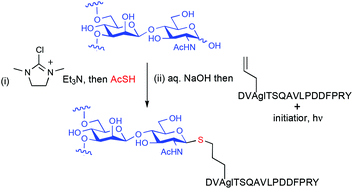
Glycosyl thiols may be accessed from the corresponding reducing sugars in water without recourse to any sugar projecting groups by way of a DMC mediated reaction with thioacetic acid in the presence of base, and hydrolysis of the anomeric thioacetate. Glycosyl thiols produced by this method may be used to access glycoconjugates, such as glycopeptides by use of the thiol–ene click reaction.


 Please wait while we load your content...
Please wait while we load your content...