Synthesis of bright red-emissive dicyanoetheno-bridged hexa-peri-hexabenzocoronene dimers†
Abstract
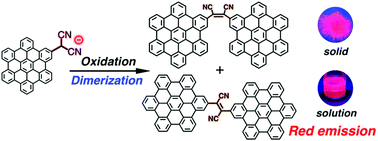
The introduction of a dicyanomethyl anion group to hexa-peri-hexabenzocoronene (HBC) substantially enhanced the emission properties of HBC due to a large perturbation of its electronic structure. In addition, dicyanoetheno-bridged HBC dimers obtained from oxidation of a dicyanomethyl HBC anion exhibited bright red emission in solution and solid states. Intramolecular charge transfer interactions between the HBC units and the dicyanoethene bridge induced solvatochromic behaviour in their emission spectra. Dicyanoetheno-bridged HBC dimers exhibited cis–trans photoisomerization behaviour in the solution, affording the mixture in cis-isomer dominance in the photostationary state. Theoretical calculations revealed that the cis-isomer is more thermodynamically stable than the trans-isomer.


 Please wait while we load your content...
Please wait while we load your content...