Photocatalytic esterification under Mitsunobu reaction conditions mediated by flavin and visible light†
Abstract
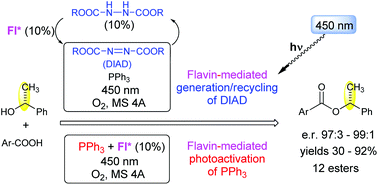
The usefulness of flavin-based aerial photooxidation in esterification under Mitsunobu reaction conditions was demonstrated, providing aerial dialkyl azodicarboxylate recycling/generation from the corresponding dialkyl hydrazine dicarboxylate. Simultaneously, activation of triphenylphosphine (Ph3P) by photoinduced electron transfer from flavin allows azo-reagent-free esterification. An optimized system with 3-methylriboflavin tetraacetate (10%), oxygen (terminal oxidant), visible light (450 nm), Ph3P, and dialkyl hydrazine dicarboxylate (10%) has been shown to provide efficient and stereoselective coupling of various alcohols and acids to esters with retention of configuration.


 Please wait while we load your content...
Please wait while we load your content...