Pd-mediated cross-coupling of C-17 lithiated androst-16-en-3-ol – access to functionalized arylated steroid derivatives†
Abstract
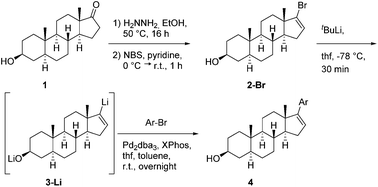
Herein, we report on Pd-mediated cross-coupling of vinyllithium steroids and aryl bromides to introduce various substituted aryls at C-17 of steroidal frameworks based on the structure of epi-androsterone. Compared to other C–C cross-couplings, this method turned out to be an easy and competitive access to biologically interesting C-17 modified steroids.


 Please wait while we load your content...
Please wait while we load your content...