Direct access to the optically active VAChT inhibitor vesamicol and its analogues via the asymmetric aminolysis of meso-epoxides with secondary aliphatic amines†
Abstract
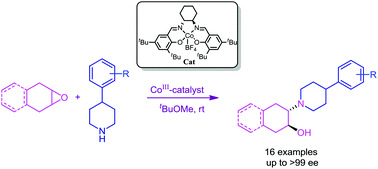
First highly enantioselective synthesis of biologically important vesamicol, benzovesamicol, and their derivatives was achieved via the desymmetrization of meso-epoxides with secondary aliphatic amines (4-phenylpiperidine derivatives) using a chiral [salenCo(III)-BF4] catalyst at room temperature. All products were obtained in good yield and with excellent optical induction.


 Please wait while we load your content...
Please wait while we load your content...