Unusual reversal of enantioselectivity in the asymmetric autocatalysis of pyrimidyl alkanol triggered by chiral aromatic alkanols and amines
Abstract
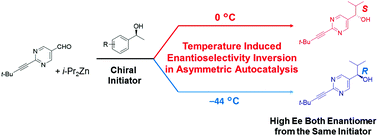
Temperature dependent inversion of enantioselectivity in asymmetric catalysis is an interesting and somewhat unusual phenomenon. We have observed temperature dependent inversion of enantioselectivity in the asymmetric autocatalysis reaction when triggered by a wide scope of enantioenriched alcohols and amines. The addition reaction of diisopropylzinc to pyrimidine-5-carbaldehyde in the presence of enantiopure alcohols or amines affords the pyrimidyl alkanol product at 0 °C with high ee. However, lowering the reaction temperature to −44 °C affords the opposite enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...