Citrate-stabilized gold nanoparticles hinder fibrillogenesis of a pathological variant of β2-microglobulin†
Abstract
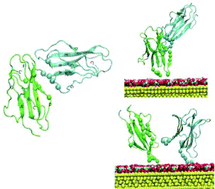
Nanoparticles have repeatedly been shown to enhance fibril formation when assayed with amyloidogenic proteins. Recently, however, evidence casting some doubt about the generality of this conclusion started to emerge. Therefore, to investigate further the influence of nanoparticles on the fibrillation process, we used a naturally occurring variant of the paradigmatic amyloidogenic protein β2-microglobulin (β2m), namely D76N β2m where asparagine replaces aspartate at position 76. This variant is responsible for aggressive systemic amyloidosis. After characterizing the interaction of the variant with citrate-stabilized gold nanoparticles (Cit-AuNPs) by NMR and modeling, we analyzed the fibril formation by three different methods: thioflavin T fluorescence, native agarose gel electrophoresis and transmission electron microscopy. The NMR evidence indicated a fast-exchange interaction involving preferentially specific regions of the protein that proved, by subsequent modeling, to be consistent with a dimeric adduct interacting with Cit-AuNPs. The fibril detection assays showed that AuNPs are able to hamper D76N β2m fibrillogenesis through an effective interaction that competes with protofibril formation or recruitment. These findings open promising perspectives for the optimization of the nanoparticle surface to design tunable interactions with proteins.


 Please wait while we load your content...
Please wait while we load your content...