Humanized archaeal ferritin as a tool for cell targeted delivery†
Abstract
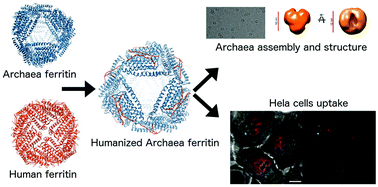
Human ferritins have been extensively studied to be used as nanocarriers for diverse applications and could represent a convenient alternative for targeted delivery of anticancer drugs and imaging agents. However, the most relevant limitation to their applications is the need for highly acidic experimental conditions during the initial steps of particle/cargo assembly, a process that could affect both drug stability and the complete reassembly of the ferritin cage. To overcome this issue the unique assembly of Archaeoglobus fulgidus ferritin was genetically engineered by changing a surface exposed loop of 12 amino acids connecting B and C helices to mimic the sequence of the analogous human H-chain ferritin loop. This new chimeric protein was shown to maintain the unique, cation linked, association–dissociation properties of Archaeoglobus fulgidus ferritin occurring at neutral pH values, while exhibiting the typical human H-homopolymer recognition by the transferrin receptor TfR1. The chimeric protein was confirmed to be actively and specifically internalized by HeLa cells, thus representing a unique nanotechnological tool for cell-targeted delivery of possible payloads for diagnostic or therapeutic purposes. Moreover, it was demonstrated that the 12 amino acids’ loop is necessary and sufficient for binding to the transferrin receptor. The three-dimensional structure of the humanized Archaeoglobus ferritin has been obtained both as crystals by X-ray diffraction and in solution by cryo-EM.


 Please wait while we load your content...
Please wait while we load your content...