Fibrillation-prone conformations of the amyloid-β-42 peptide at the gold/water interface†
Abstract
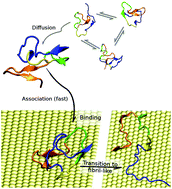
Proteins in the proximity of inorganic surfaces and nanoparticles may undergo profound adjustments that trigger biomedically relevant processes, such as protein fibrillation. The mechanisms that govern protein–surface interactions at the molecular level are still poorly understood. In this work, we investigate the adsorption onto a gold surface, in water, of an amyloid-β (Aβ) peptide, which is the amyloidogenic peptide involved in Alzheimer's disease. The entire adsorption process, from the peptide in bulk water to its conformational relaxation on the surface, is explored by large-scale atomistic molecular dynamics (MD) simulations. We start by providing a description of the conformational ensemble of Aβ in solution by a 22 μs temperature replica exchange MD simulation, which is consistent with previous results. Then, we obtain a statistical description of how the peptide approaches the gold surface by multiple MD simulations, identifying the preferential gold-binding sites and giving a kinetic picture of the association process. Finally, relaxation of the Aβ conformations at the gold/water interface is performed by a 19 μs Hamiltonian-temperature replica exchange MD simulation. We find that the conformational ensemble of Aβ is strongly perturbed by the presence of the surface. In particular, at the gold/water interface the population of the conformers akin to amyloid fibrils is significantly enriched, suggesting that this extended contact geometry may promote fibrillation.


 Please wait while we load your content...
Please wait while we load your content...