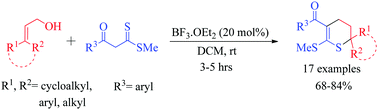
Synthesis of polysubstituted 3,4-dihydro-2H-thiopyrans by regioselective annulation of 3,3-disubstituted allylic alcohols with a β-oxodithioester†
Abstract
Polyfunctionalized 3,4-dihydro-2H-thiopyrans were obtained in high yields via the regioselective annulation of 3,3-disubstituted allylic alcohols with a β-oxodithioester under BF3·Et2O catalysis.


 Please wait while we load your content...
Please wait while we load your content...