Conformational behaviour, photochemistry and flash vacuum pyrolysis of a 2-(1H-tetrazol-1-yl)thiophene†
Abstract
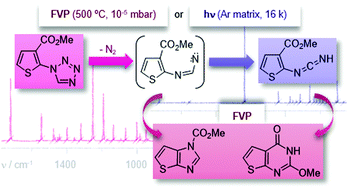
The conformational properties and the photochemical behaviour of a 2-(1H-tetrazol-1-yl)thiophene were studied using low-temperature matrix isolation (argon; 16 K) coupled with infrared spectroscopy. The experimental results were supported by theoretical calculations carried out at the B3LYP/6-311++G(d,p) level. The calculations predicted two conformers for the studied compound in the gas phase that differ in energy by 3.2 kJ mol−1. Accordingly, two conformers were observed in the low-temperature matrix. UV-laser irradiation at 245 nm of the studied compound isolated in the argon matrix led to photocleavage of the tetrazole ring, with elimination of N2 and formation of the corresponding thiophen-2-ylcarbodiimide, most likely via the corresponding imidoylnitrene intermediate. The flash vacuum pyrolysis of 2-(1H-tetrazol-1-yl)thiophene gave methyl 1H-thieno[2,3-d]imidazole-1-carboxylate via intramolecular formal insertion into a C–C bond of the corresponding imidoylnitrene intermediate and the unexpected 2-methoxythieno[2,3-d]pyrimidin-4(3H)-one. The synthesis of this heterocycle was rationalized considering the initial N2 elimination to generate an imidoylnitrene followed by rearrangement to the corresponding thiophen-2-ylcarbodiimide, [1,5] sigmatropic shift of the methoxyl group and electrocyclic ring closure.


 Please wait while we load your content...
Please wait while we load your content...